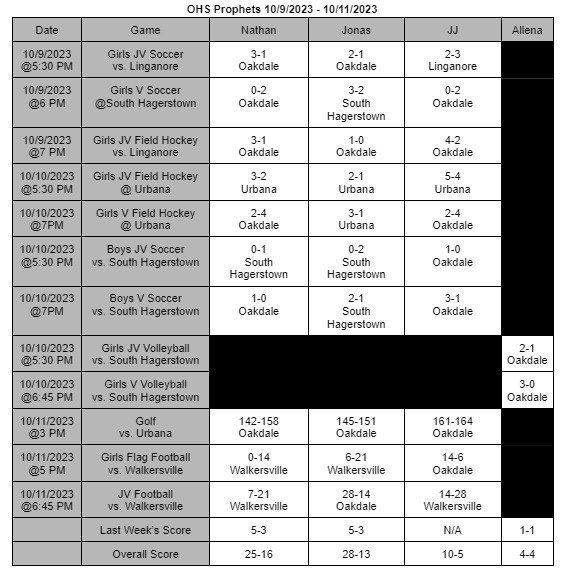
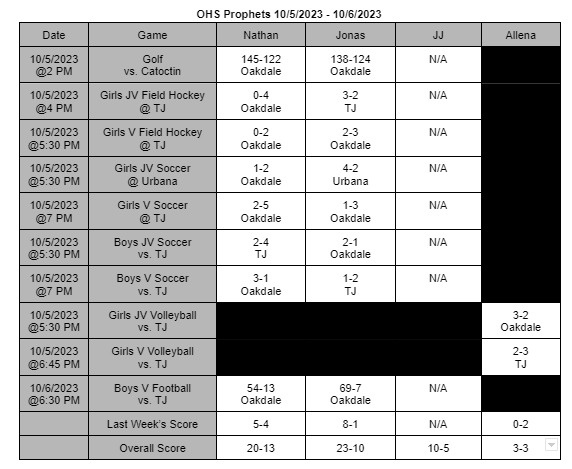
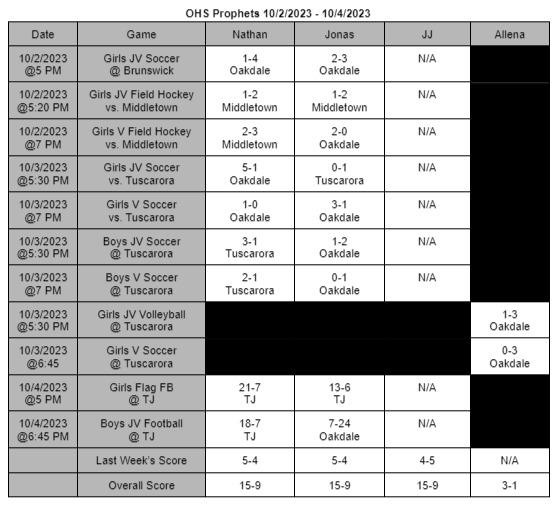
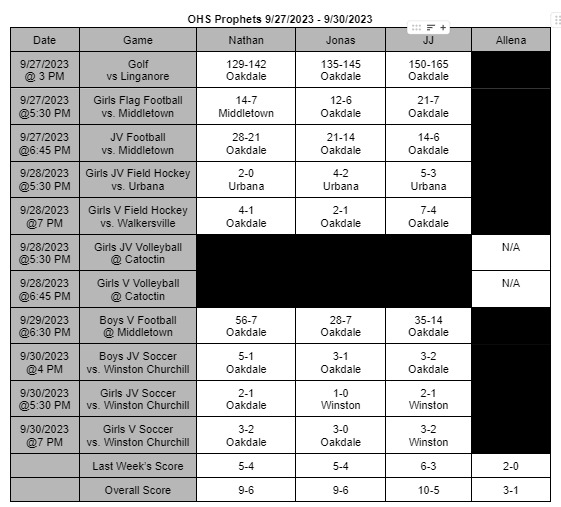
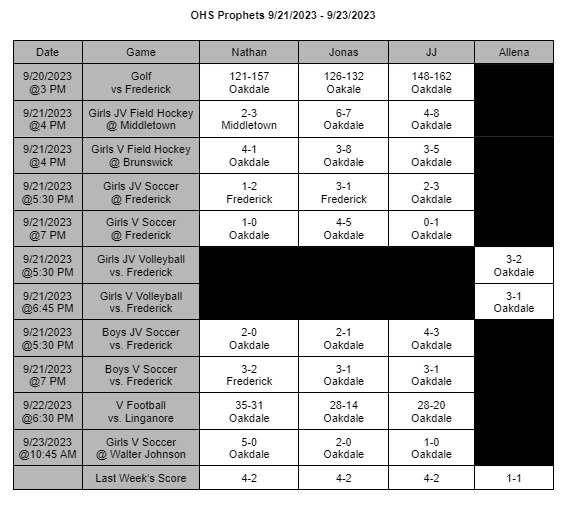
The FDA finds a contagion in Zantac Heartburn medication and declares a recall of all items in stores
April 11, 2020
On April 1st, 2020 the FDA ordered all products of Zantac Heartburn medicine to be removed from shelves. The products were found to have a contagion known as N-Nitrosodimethylamine (NDMA), also known as ranitidine. The contagion grows more deadly the longer it is left sitting; not only that, the contagion can grow if left in areas hotter than room temperature.
The medicine was under close watch for about a month and the World Health Organization (WHO) classifies this drug as a possible human carcinogen. In other words, it’s a substance capable of causing cancers in any living tissue of the body. The WHO highly recommend anyone who takes it or any form of Zantac stop immediately.
The Food and Drug Administration (FDA) has stated, “The impurity in some ranitidine products increases over time and when stored at higher than room temperatures,” the FDA wrote in a news statement. In other words, the longer the medicine sits in hotter temperatures, the more contagion is made in the medicine: which increases the chances of cancer formations. It’s currently unknown how long certain boxes have been on shelves causing confusion and panic to people who take it.
The FDA also continued, “It’s an exposure to unacceptable levels of impurity.”
“Since we don’t know how or for how long the product might have been stored, we decided that it should not be available to consumers and patients unless its quality can be assured,” stated Dr. Janet Woodcock, the director of the evaluation and research department. Woodcock continues by stating in a press release.
“We set out to fully understand this issue and provide actionable information for Americans who use these medications. The information we’ve gathered as part of our ongoing ranitidine investigation has been vital to answering the questions we’ve received about the potential risk of these products.” Woodcock’s team will be putting out new information as soon as it’s released on the FDA’s website, https://www.fda.gov/.
The underlying message is to avoid any medicine that contains this substance for it can cause you harm or possibly cancer. There are many alternatives such as TUMS or Nexium you can adapt to. If these medicines conflict with any personal or medical situations it’s best to contact your doctor as soon as possible.
Photo Caption: Picture taken of CVS stores while recall was taking place, 4/1/20.